The House Oversight Committee is opening an investigation into the Trump administration’s deployment of rapid COVID-19 tests to nursing homes around the country after TPM revealed in August that the tests had been sent without warning or guidance on their operation.
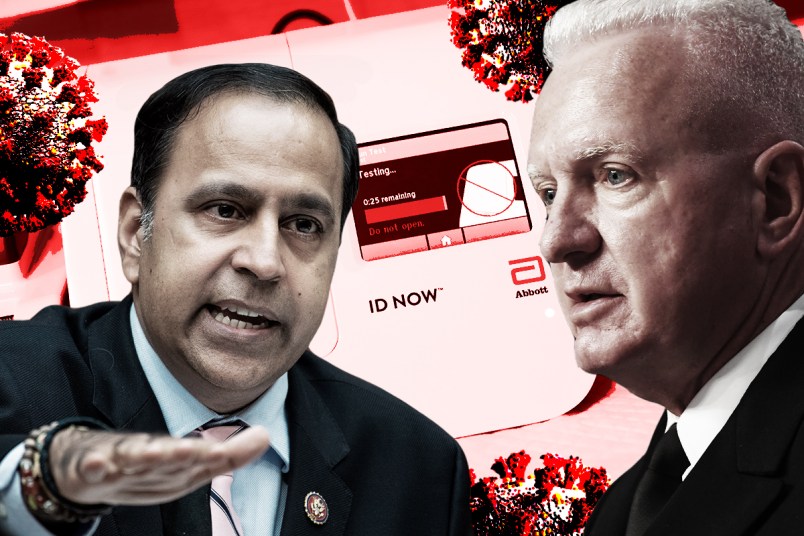
Rep. Raja Krishnamoorthi (D-IL), the chairman of the House Oversight Subcommittee on Economic and Consumer Policy, said in a letter Friday that he is probing allegations that the Department of Health and Human Services distributed thousands of testing kits in a reckless manner, failing to provide guidance to recipients.
The Krishnamoorthi letter has not previously been reported.
The result, TPM reported originally, was mass confusion, the potential for underreporting of test results, and conflict with state authorities that had banned the use of the rapid antigen tests in nursing homes because of their lower accuracy.
The subcommittee announced the probe in an Oct. 2 letter to HHS Assistant Secretary for Health Adm. Brett Giroir, who oversaw the ambitious program in which HHS and the Centers for Medicaid and Medicare Services would supply every nursing home in the country with a rapid testing kit.
“The Subcommittee is concerned that HHS is not providing clear guidance on how to use these antigen tests, resulting in widespread confusion,” Rep. Krishnamoorthi wrote in the letter. “When used improperly, tests can create dangerous situations. Epidemiologists have shared a concern with me that if tests with insufficient sensitivity are used to ‘screen’ visitors to nursing homes, false negatives risk allowing entry to contagious persons who could seed a deadly outbreak.”
The antigen tests make a limited trade between speed and accuracy, providing less reliable results within a 15-minute timeframe. Scientists told TPM in August that the tests were best used and most accurate when testing people who were already showing symptoms that could be consistent with COVID-19 or other illnesses.
But when HHS announced the initiative on July 14, the agency stated that it would give nursing homes the ability to “screen and test” residents and visitors. That, Krishnamoorthi wrote in the letter, appears to conflict with the devices’ instructions, which say that the antigen tests “should not be used for screening of
asymptomatic individuals.”
As confusion over the testing kits spread this summer, multiple officials with local nursing homes and national organizations representing long-term care facilities and state epidemiologists told TPM about the problem.
Some of these organizations drafted their own guidance to help nursing homes figure out in what situations to use the tests and how to report the results. Federal law has mandated since March that all COVID-19 test results must be reported to public health authorities.
Dr. Jeffrey Engel, a senior advisor on COVID-19 and an executive at the Council of State and Territorial Epidemiologists, told TPM in August that he had petitioned Giroir directly about the problem.
“We’ve been asking for federal guidance all along, these nursing homes have very limited capacity, and to expect them to ramp up and do this — something that they’ve never done before — is not realistic and could be potentially harmful,” Engel said.
Engel also said at the time that Giroir had failed to provide a list of nursing homes that received the rapid testing kits.
An HHS spokeswoman told TPM in August that the responsibility to instruct nursing homes on how to use the tests lay with the manufacturers. “Training documentation will be made widely available for all nursing homes that are receiving supplies,” the spokeswoman said.
The administration eventually expanded the program to distribute the rapid testing devices to schools and other areas where COVID-19 infection is likely, signing a $750 million deal with Abbott Laboratories. The terms of the devices’ procurement are also under investigation.
Rep. Krishnamoorthi demanded in his letter — sent to HHS Friday morning — that HHS provide by Oct. 16 a list of all the nursing homes that received the testing kits. He is also seeking all published and draft guidance that HHS provided to recipients of the tests, information about how recipients were prioritized, internal communications about the program, and documents surrounding the procurement of the testing devices.
The lawmaker added in the letter that the lack of guidance had made it harder for nursing homes and schools to design testing plans “to effectively control the spread of coronavirus.”
“The Subcommittee seeks your commitment that, as HHS ramps up its antigen test deployment to a massive scale with the procurement of 150 million more antigen tests, it will provide clear and effective guidance to nursing homes, schools, states, and communities on how to use them appropriately,” the message reads.
Read the letter here:






Another “macaca” moment?
Kudos!
And whatever the oversight committe does will be ignored.
Kudos to TPM. Go team!
We must stay vigilant and watch everything that is done by this Konniving Klan of Kriminals.
Krishnamoorthi is a Democrat.
I want a probe into which five languages she is supposedly “fluent” in.
Melania Trump also is heard saying on the tape that many migrant children with families seeking asylum are “teached by other people what to say” to stay in the U.S.