Last month, Dr. Michael Wasserman received a surprise at the nursing homes he operates.
It came in the form of a delivery from the Department of Health and Human Services: three rapid COVID-19 testing machines sent to nursing homes that Wasserman, president of the California Association for Long Term Care Medicine, directs.
Part of Wasserman’s surprise stemmed from the fact that he had no idea the machines were coming without instruction. Neither, for that matter, did the state government, which had regulations on the books that barred the use of the machines in California nursing homes.
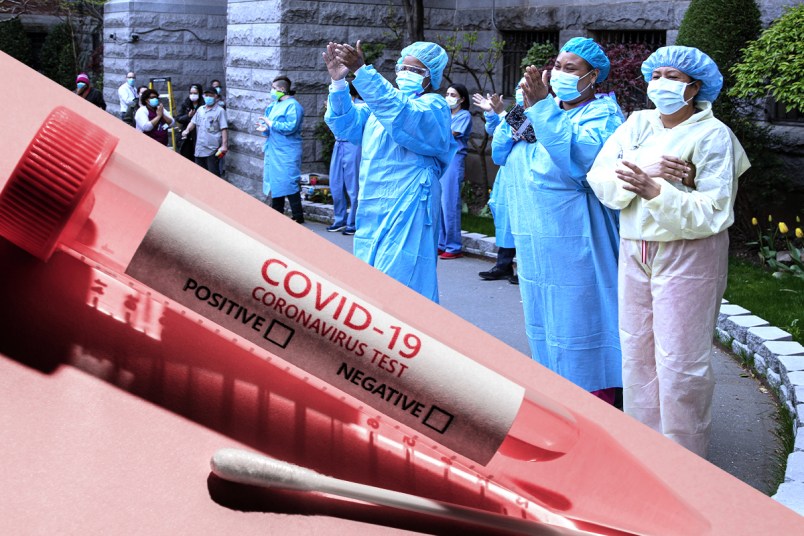
As he sought answers, Wasserman encountered an HHS program to surge rapid, point-of-care COVID-19 testing machines to nearly every nursing home in the country. Long awaited by nursing homes, the program relies on a technology called antigen testing that, in its current form, trades accuracy for a 15-minute return on results.
But the HHS program to distribute the machines has been met with mass confusion stemming from a lack of direction over what to do with the machines and how, or whether, to record and report the results. As a result, some nursing homes have been failing to report the results of tests to state health departments. The tests’ accuracy has also been reduced by operator error, clouding the true spread of COVID-19.
“I have to say, I’m not impressed that they basically sent us machines without clear direction on how to use them, and without clear direction to state and county health departments,” Wasserman said.
Wasserman is not alone in his concern.
“HHS needs to own that — they’ve got to send out [the testing machines] with explicit responsibilities for reporting results to public health authorities and technical assistance so it actually happens,” said Dr. Jeffrey Engel, a senior advisor on COVID-19 and executive at the Council of State and Territorial Epidemiologists.
“We’ve been asking for federal guidance all along, these nursing homes have very limited capacity, and to expect them to ramp up and do this — something that they’ve never done before — is not realistic and could be potentially harmful,” Engel told TPM.
Engel, whose organization represents state epidemiologists around the country, told TPM that his group’s members were contending with nursing homes that were not reporting their test results. When CSTE reached out to HHS for a list of what machines had gone to which nursing homes in a bid to determine where testing on the machines was occurring, Engel said, HHS rebuffed him.
This lack of clarity will likely “impact disease control overall,” said Dr. Cyrus Shahpar, a former commander in the U.S. Public Health Service and director of the Epidemic Intelligence Unit at the public health organization Vital Strategies.
He said that if nursing homes were unaware of reporting requirements, it would make it harder for state and local public health departments to track the outbreak.
“To do that, you need to know where the disease is, where it’s spreading, where the gaps are,” Shahpar said. “We’ll be put in a position where we might not find out about these things until they’re extensive, and have delayed recognition of pockets of disease.”
An HHS spokeswoman told TPM that the manufacturers would provide instructions and videos to train staff on the devices.
“Training documentation will be made widely available for all nursing homes that are receiving supplies,” the spokeswoman said.
The American Health Care Association, the national nursing home lobby, told TPM that it was advising its members to follow state and local public health guidelines to use the tests.
“CMS and HHS recognized that providers will have many questions, but they did not want to delay in getting this program started and will be providing more guidance in the coming weeks,” a spokesperson said.
Rapid tests, lower accuracy
HHS announced the ambitious project last month with the Centers for Medicare & Medicaid Services: the agencies would send rapid COVID-19 testing machines to every nursing home in the country. The tests deliver on-the-spot results, but are prone to false positives if used correctly, and false negatives if there’s operator error or if the test is conducted too early in COVID-19’s progression.
“Where there’s discrepancies, often is in user error rather than the test being faulty,” Joe Petrosino, chair of molecular virology at Baylor University, told TPM. He added that sample collection for the approved antigen tests require swabs deep in the nasal cavity.
From the Trump administration’s standpoint, the machines could help test a population that has accounted for 40 percent of U.S. deaths in the pandemic. It could also resolve a key issue: unacceptably long turnaround times on testing. Nursing home staff in states with COVID-19 outbreaks must be tested for the disease once per week, adding to the burden placed on large, commercial laboratories. Antigen tests don’t rely on such laboratories, and provide results on the spot.
“By putting point-of-care [testing] in nursing homes we not only improve turnaround and protect our vulnerable there, but we offload a lot of the demand on the major laboratories,” HHS Assistant Secretary for Health Adm. Brett Giroir told reporters last month.
Many nursing home operators feel similarly. Used correctly, the tradeoff between accuracy and speed allows caregivers to quickly identify a potential COVID-19 outbreak.
“It’s a choice between the sensitivity of the test and the timeliness of the results,” Kevin Warren, CEO of the Texas Health Care Association, which represents nursing homes in the state, told TPM.
So, who is testing?
HHS is distributing the only two antigen testing machines approved for use right now on the market. The tests showed a roughly 80 percent sensitivity rating in controlled, pre-market tests.
But there’s a problem: it’s not clear how, or if, many of the results are being reported. HHS did not inform the nursing homes that received the machines of their obligation to report the results, and without federal guidance, it’s also not clear how states should account for the lower accuracy of antigen tests in the cases where the test results are reported.
In Iowa, a Van Buren county public health official told TPM that she elected to report her county-level antigen positive test results publicly on the county Facebook page even though her state did not release them to the public, while a Florida test provider told TPM that his local public health department had initially refused to accept antigen results.
In Vermont, the state’s department of public health told TPM that it would soon issue “a reminder about reporting results to the Health Department,” while Warren, the Texas nursing home representative, described the lack of reporting as a “challenge.”
“You can’t fight what you can’t see,” he said.
Dr. Wasserman, the Los Angeles nursing home director, told TPM that the lack of clear guidance was particularly difficult given that the the CARES Act mandates that all COVID-19 tests be reported to state authorities.
“This is one of the challenges with nursing homes, they’re running around trying to report to god knows how many different authorities,” Wasserman said. “It would really have helped if we had clear direction from the federal government, one that gave the state and local health departments an opportunity to follow that direction.”
It’s impossible to quantify the scale of the problem, in part because, according to Engel, HHS has refused to share information on which nursing homes have received the equipment so far. Though HHS says that it intends for every nursing home in the country to have a rapid, point-of-care antigen test by the end of September, it’s not clear in what order the tests are being delivered.
In California, the confusion was also compounded because the antigen tests failed to meet California requirements — more stringent than those in other states — on the sensitivity of tests in nursing homes. It’s not clear how many tests were delayed because of this, though the California Department of Health told TPM it ultimately allowed the machines to be used. CMS records show that 265 separate nursing homes in California are set to receive the antigen machines.
Dr. Susan Butler-Wu, an associate professor of clinical pathology at USC and the director of a microbiology lab, described confusion over reporting antigen test results as part of a disorganized national strategy that has been an “unmitigated disaster.”
“The virus doesn’t care what state it’s in — it’s a virus,” she said. “You have to have a standardized national approach, because we are one nation with open borders across our states.”
To Wasserman, the lack of direction and resulting confusion came down to a lack of federal leadership.
“A pandemic that kills the number of nursing home residents that this pandemic has killed requires clear federal direction,” he said.






My, how completely unexpected and out of character…
Pet project update:
With 132 days until election eve 581 deaths per day were required to hit the 200k milestone the morning people trekked to the polls.
Today, it’s 76 days until election eve. Per Johns-Hopkins tallies we need to only average 370 deaths per day to hit the 200k milestone.
Anyone tells you Trump is getting this under control is full of shit. The numbers don’t lie.
Criminal negligence. A government job doesn’t mean “try your best to see if you can get anything done, but if you can’t that’s fine.” You have responsibilities in line with your unit’s mission – and no one in this administration has the training or, honestly, the aptitude, to meet those responsibilities. History will not be kind to these homicidal nimrods.
Trump will have his fair share of Holocaust deniers in his corner in future generations. Dumb dies a hard, protracted death.
Wait. Most nursing homes don’t usually have lab techs, do they, to run such tests? They might do blood draws, and send them to a lab, I’d think. I suppose it depends on the nursing home.
(And I’m sort of lost by use of the term “instruction,” as here “sent with no instruction.” Normally, we call them “instructions.” But maybe they mean training as well as a manual, as in RTFM. Perhaps being picky, but that’s my job.)