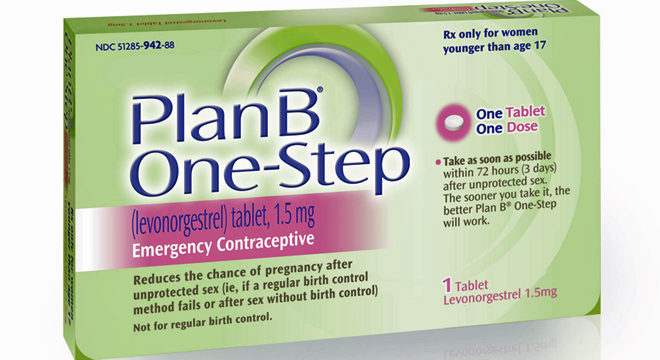
The Food and Drug Administration said Monday that it will review data on emergency contraceptive drugs after reports that a similar European drug, Norlevo, changed its labeling due to research that it was ineffective on women who weigh more than 176 pounds, according to Mother Jones.
“The FDA is currently reviewing the available and related scientific information on this issue, including the publication upon which the Norlevo labeling change was based,” FDA spokeswoman Erica Jefferson wrote in an email to Mother Jones. “The agency will then determine what, if any, labeling changes to approved emergency contraceptives are warranted.”