There’s never been a better time to be in the molecular imagery business. On the heels of IBM’s announcement in late February that it managed to get the first-ever images of an electrical charge distribution throughout a molecule, scientists at Ohio State University and the Kansas State University announced this week that they’d gone one step further and snapped the first ever pictures of atoms moving in a single molecule.
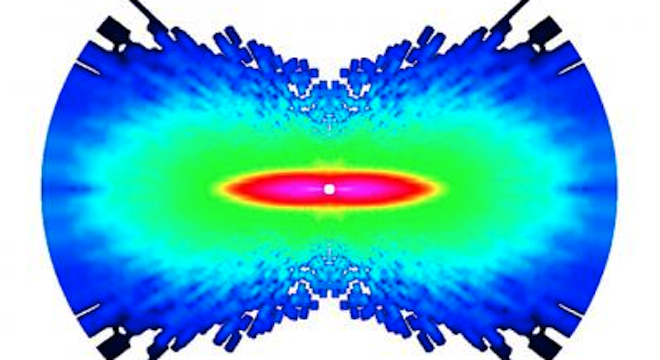
Specifically, the scientists managed to get images of atoms vibrating in oxygen and nitrogen molecules by shooting laser pulses at them and knocking one of each of their electrons out of orbit. When the electron fell back into the molecule, it scattered off its ions similar to the way a flash scatters light off of the subject of a photo, allowing researchers to record the atoms vibrating. The team published their findings in the journal Nature on March 7.
However, as one of the paper’s authors, OSU post-doc Cosmin Blaga, told TPM, the actual technique used has been around for quite some time.
“As is often the case in science, most ideas float around before a discovery is made,” Blaga said in an email. “The rescattering mechanism was well known since early 90s. People knew that electrons scatter on their parent ions.”
Blaga said that the team’s main innovation — and the reason why nobody had achieved such a view of atomic vibrations before — was overcoming the molecule’s electrical field, which would block out any imagery of the scattering. Blaga told TPM that the team was able to address this problem by using laser pulses in the mid-infrared range, rather than the near or far range.
Intriguingly, the team managed to get the images right on the first try.
“We knew it was working already,” Blaga said, thanks to the computing models run by the team at Kansas State. The actual experiment itself took place at the Physics Research Building at The Ohio State University.
The resulting colorized images show atoms vibrating in the time in between laser pulses, one quadrillionth of a second. The pink regions are those with the most movement, decreasing to blue, those areas with the least motion. The molecule pictured at the top of this post is nitrogen.
Perhaps most intriguing of all is what happened to the molecules once one of their atom’s electrons was knocked of its orbit to create the image.
As Blaga explained: “Most molecules simply became ions, milliseconds later capturing back an electron from collisions with the vacuum chamber’s stainless steel walls. Few molecules broke apart…The gas pressure inside the chamber [where the experiments were run] was a million times lower compared to the air we breathe, so molecules didn’t have neighboring molecules to readily pair up, so no chemistry was observed in this case.”
But in the long term, Blaga hopes that the imagery will be used to capture more complicated chemical interactions and then actually control those chemical reactions on the molecular level, enabling a whole new class of materials that don’t exist in nature or anywhere in the known universe, for that matter.
“What we ultimately want to do is tell the compound how and into what to break apart,” Blaga said. “We’re not there yet, but we think that laser driven electron diffraction is a valuable tool for this purpose.”